Combing through huge medical databases to arrive at an accurate prognosis can now be entrusted to machines and verified by experts.
In the field of genomic medicine, clinicians need to know how any genetic mutations in a patient have the ability to cause disease. If such a mutation is found, clinicians can then consider a treatment for that specific mutation.
However, only a small percentage of the vast number of possible genetic mutations has been linked to disease. Also, there are genetic mutations with unknown potential to cause disease, but that does not mean they do not directly or indirectly do so.
Now, this genetic research conundrum is being tackled with the use of AI and machine learning. Fujitsu and Kyoto University have now developed a system that can estimate the presence or absence of disease-causing potential for unknown genetic mutations and explain the basis for this finding.
How the system works
When data about a genetic mutation is input into the system, the information is cross referenced to a huge database of existing evidence from previous research. Machine learning algorithms trained on the big data can arrive at various conclusions, and the workings behind these findings are always explained, as part of what is known as ‘explainable AI’.
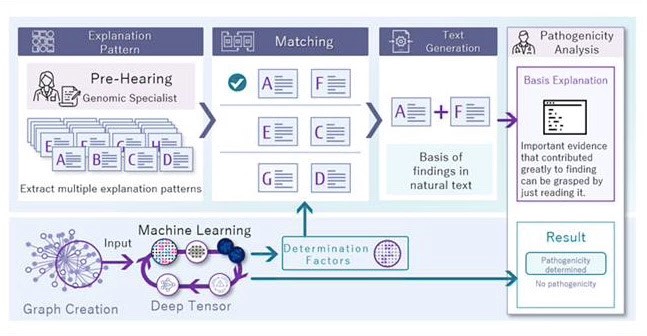
In addition, the system also makes it easy for medical professionals and researchers to use existing public databases to arrive at their own estimations and findings—by generating a multi-faceted visualization of relevant data and literature onscreen. A clinician can then use the system’s AI features such as natural language processing to speed up literature reviews and research techniques to come to useful conclusions about the mutation being analyzed.
The three innovations in this system are:
- Deep tensor machine learning
Based on information from public databases related to genetic mutations, ‘Gene Mutation Knowledge Graphs’ are created to serves as machine learning training data. In constructing the graphs, a group led by Professor Yasushi Okuno of Kyoto University define an effective graph range for estimation, and using Fujitsu’s ‘Deep Tensor’ machine learning technology, clinicians aided by AI features can easily use their insights and intuition to complement the AI-generated clues to reach an educated estimate regarding a particular gene mutation’s potential for causing diseease. - AI-enhanced research features
How the system arrives at various findings is always explained in natural language that humans can understand. This can help researchers to spot AI biases or any potential problems in the system-generated intelligence.
Also, as the Deep Tensor technology used to arrive at findings can be extremely complex, much research has been done to enhance the ‘explainable AI’ to help medical professionals and researchers understand the important evidence that was used for the machine-generated estimations.
- Enhancing verification of machine findings
As machine learning becomes more accurate as it trains on ever-more data being fed into it, its accuracy depends on the data being use. As such, the system’s results need to be cross-verified by humans. This process can be tedious due to the huge amount of data and academic literature required to be checked.
To assist humans in this process, the system not only generates clear explanations of how its conclusions were arrived at, but also aids humans in performing their own verification research. In addition to displaying multifaceted information on a single screen for quick reading and review, the system can be set to highlight only the most relevant passages or data. Searching is also enhanced via AI through natural language processing.
With the cooperation of the Human Genome Center of the Institute of Medical Science at the University of Tokyo, an evaluation test of the system, called ‘MGeND Intelligence’, produced impressive results. For example, the pathogenicity of one mutation was unknown at the time of the creation of the verification system used in the evaluation test, and was later judged to be pathogenic. In this case the pathogenicity estimation AI made a correct estimation of the mutation’s pathogenicity.
Additionally, the evaluators were able to obtain an overview of the basis of the AI’s findings by reading the AI-generated explanatory text, and to gather sufficient evidence to consider that it was pathogenic.
Ultimately, the use of this MGeND Intelligence offers the potential to deliver greater innovations in medical care for areas including treatment planning for genomic therapies for illnesses like cancer, accelerating the optimization of patient-centric medical care.
